Abstract

Patients with hematologic malignancies (HM) are immunocompromised and have worse clinical outcomes with COVID-19 infection than the general population. As previously reported (Niu et al. Leukemia Research 2021), this may in part be due to an altered immune system that is different from that of immunocompetent hosts. Therefore, it is imperative that patients with HM receive additional protection from COVID-19. However, with a distorted immune system, the question of whether patients with HM can mount an appropriate antibody response (AAR) is unknown. Here, we evaluated the antibody response to the COVID-19 vaccine in previously infected and uninfected patients with HM.
From 3/31/20 to 05/31/22, we examined 400 patients (age 18 to 86), 120 of which had a HM (58 lymphoid, 53 multiple myeloma, and 9 myeloid), 58 had a solid malignancy (SM), and 222 were controls (without a HM or SM). These 400 patients had their 1st and 2nd vaccinations, 3rd vaccination/booster, and 4th vaccination/2nd booster, (if all or any were applicable) documented. Following informed consent in accordance with the Declaration of Helsinki and IRB approval, blood draws were obtained with respect to each vaccination and/or booster date to assess antibody response. To standardize antibody response in this analysis, only patients that had antibodies collected after 90 days from their specific vaccination/booster were analyzed. The main antibodies evaluated were the SARS-CoV-2 virus antibodies toward spike proteins (anti-spike). Anti-N and anti-S antibodies were also obtained for future analysis. An AAR was determined to be an anti-spike level of greater than 0.5. If patients had a positive anti-S response (>0.5), they were considered as having been previously infected regardless of clinical history.
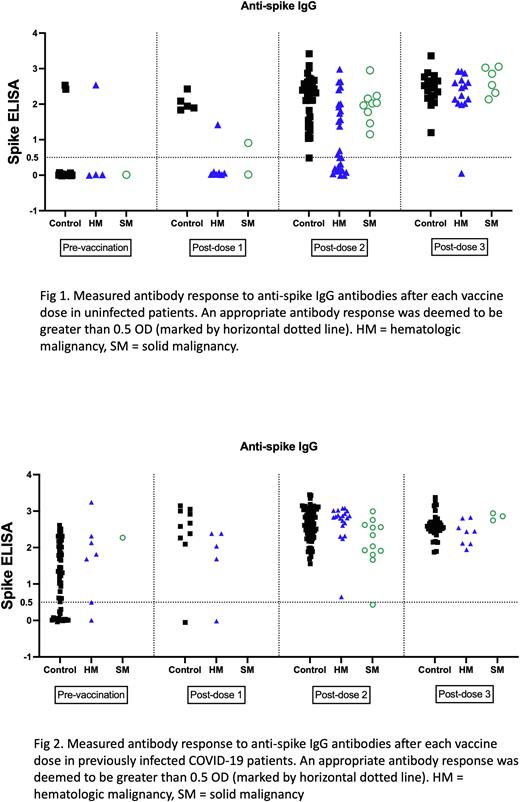
In patients without prior COVID-19 infection, analysis of antibody levels post-1st vaccination showed all evaluated control patients (5 of 5) as developing an AAR, while only 1 of 7 HM patients and 1 of 2 SM patients developed an AAR. Analysis after the 2nd vaccination showed most control patients (27 of 28) developing an AAR and all patients with SM developing an AAR (9 of 9). However, only 50% of HM patients developed an antibody response (14 of 27). After the 3rd injection/booster, all control group patients (18 of 18), all SM patients (6 of 6), and most HM patients (13 of 14) developed an AAR (Fig 1). A subgroup analysis for the post-dose 2 vaccination in HM patients found that for non-multiple myeloma lymphoid malignancies, 5 of 11 patients didn't develop an AAR. 4 of these patients had received treatment for their underlying malignancy within 3 months of the vaccine. For the 6 responders, 5 of these patients were not on any treatment. In patients with prior COVID-19 infection, post-1st vaccination analysis showed almost all control patients as developing an AAR (9 of 10) while 4 of 5 patients with HM responded. No SM patients were collected. After the 2nd vaccination, all control patients (71) and all HM patients (20) had an AAR, while 11 of 12 SM patients had an AAR. Following the 3rd injection/booster, all evaluated patients (33 control, 7 HM, and 3 SM) had an AAR (Fig 2).
Our evaluation of anti-spike antibodies in response to the COVID-19 vaccine in patients with HM, showed that this specific group responded differently than the general population or those with SM. In our uninfected cohort, only half of the evaluated HM patients had an AAR after the 2nd vaccination, while most control and SM patients had the desired response. In comparison, HM patients who had a COVID-19 infection developed an AAR after their 2nd vaccination. This indicates that patients with HM likely require additional immune activation before achieving an AAR. Close to half of HM patients in the uninfected cohort required 3 vaccine doses to achieve an AAR. In previously COVID-19 infected HM patients, the majority were able to achieve an AAR after 2 vaccinations, indicating exposure to the virus may have helped provide additional immunity. Thus, as the landscape of the COVID-19 pandemic is constantly shifting, the current guideline of vaccines and their efficacy in immunocompromised patients is something that will need to be consistently addressed. As more data is collected on our cohort of patients, we will track the longitudinal antibody responses (anti-spike, anti-N, and anti-S) for each patient over time, overall trends of antibody response after the 4th vaccination, and T-cell responses.
Disclosures
Safah:Astellas: Speakers Bureau; Janssen: Speakers Bureau; Incyte: Speakers Bureau; Blueprint: Speakers Bureau. Saba:ADC Therapeutics: Other: Advisory Board; Pharmacyclics: Other: Advisory Board, Speakers Bureau; AbbVie: Consultancy, Other: Advisory Board, Speakers Bureau; Janssen: Other: Advisory Board, Speakers Bureau; Kyowa Kirin: Other: Advisory Board.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal